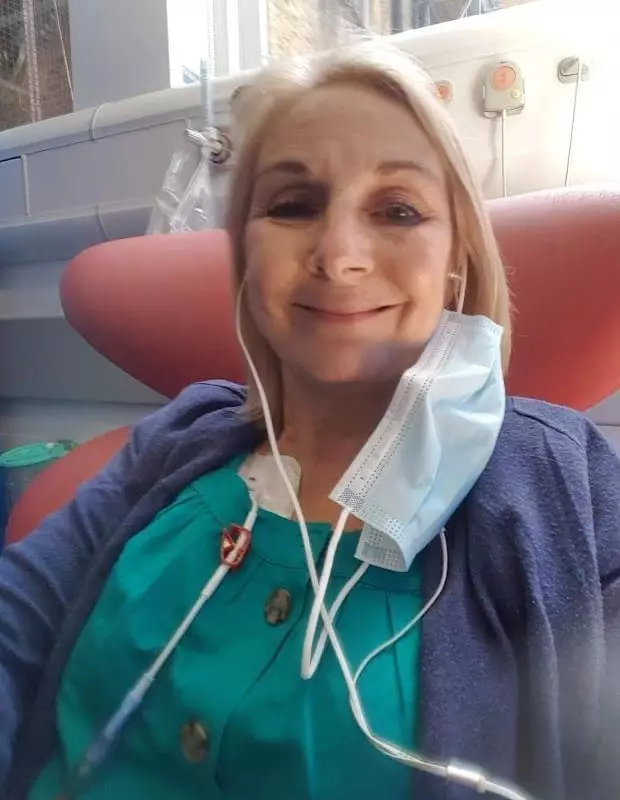
A Birmingham grandmother is celebrating a monumental victory after the life-extending cancer treatment that gave her a future was officially approved for use on the NHS.
A Long Road to Diagnosis
Sue Harley, now 63, was just 54 when she received a diagnosis of the incurable blood cancer myeloma in 2017. Her journey to this point was fraught with difficulty, beginning in 2015 with increasingly debilitating back pain.
For two years, her symptoms were mistakenly attributed to osteoarthritis and old age. The situation deteriorated to the point where she struggled to walk and was once found asleep in her local library due to sheer exhaustion. After scans around Christmas 2016 revealed holes in her spine, she was initially, and incorrectly, diagnosed with metastatic breast cancer before doctors identified the true culprit: myeloma.
Finding Hope in a New Treatment
Over the subsequent six years, Sue underwent five consecutive treatments, but none managed to keep her cancer at bay for long. By 2023, she had exhausted the standard options.
Her fortunes changed when she gained access to a next-generation drug called talquetamab through a compassionate use programme. This groundbreaking treatment is part of a new class of bispecific antibodies that work by binding to both myeloma cells and the immune system's T cells, directing them to destroy the cancer.
Two years on, Sue is in her longest remission. "It has absolutely given me my life back," she said. "I feel fit and healthy, I've run two half-marathons since I've been on talquetamab. I look after my grandchildren – they're a joy."
A Campaign for Wider Access
Determined to help others, Sue joined forces with the charity Myeloma UK to campaign for the drug's approval by the National Institute for Health and Care Excellence (NICE).
Their hard-fought effort paid off when NICE gave talquetamab the green light on Monday 17 November. This decision means up to 800 patients each year in England and Wales with heavily pre-treated myeloma are expected to benefit.
Shelagh McKinlay, Director of Research and Advocacy at Myeloma UK, hailed the decision: "Talquetamab offers more flexibility and leads to a better quality of life... This is a hard-earned victory for patients." The charity continues to push for approval in the rest of the UK.
For Sue, the approval represents more than just a treatment; it represents time. "When I was diagnosed, I didn't know if I would live to see grandchildren, let alone live to see them grow up," she reflected. "This gives people a chance to live their lives, just like everybody else."